
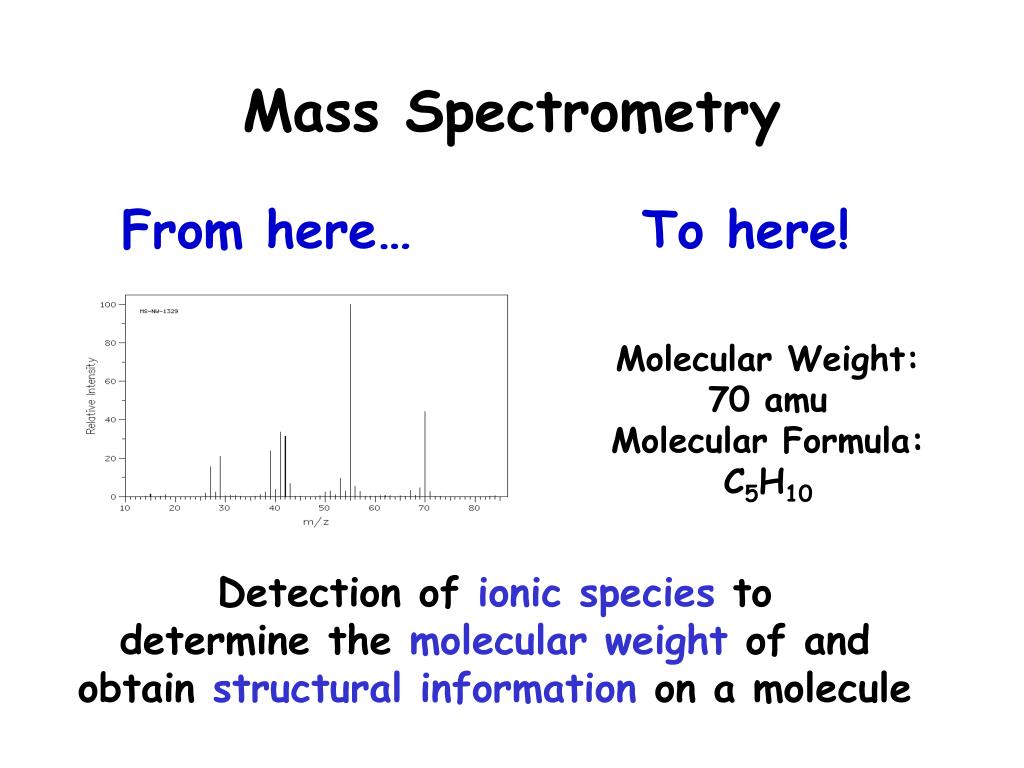
In that case, you would get a strong line at 57. With pentan-3-one, you would only get one ion of this kind: That would give you strong lines at m/z = 43 and 71. In the pentan-2-one case, there are two different ions like this: pentan-2-oneĮach of these is likely to split to produce ions with a positive charge on the CO group. Suppose you had to suggest a way of distinguishing between pentan-2-one and pentan-3-one using their mass spectra. Using mass spectra to distinguish between compounds In these two spectra, this is probably the most dramatic example of the extra stability of a secondary carbocation. You would get the same ion, of course, if the left-hand CH 3 group broke off instead of the bottom one as we've drawn it. Again a secondary carbocation is formed - this time, by: The peak at m/z = 57 is much taller than the corresponding line in pentane. The ion formed is a secondary carbocation - it has two alkyl groups attached to the carbon with the positive charge. This peak in 2-methylbutane is caused by: This is caused by a different ion than the corresponding peak in the pentane mass spectrum. Look first at the very strong peak at m/z = 43. 2-methylbutane is an isomer of pentane - isomers are molecules with the same molecular formula, but a different spatial arrangement of the atoms. Let's look at the mass spectrum of 2-methylbutane. A split producing a tertiary carbocation will be more successful still. Summarizing the most important conclusion from the page on carbocations:Īpplying the logic of this to fragmentation patterns, it means that a split which produces a secondary carbocation is going to be more successful than one producing a primary one. For example, the mass spectrum of pentane looks like this: All sorts of fragmentations of the original molecular ion are possible - and that means that you will get a whole host of lines in the mass spectrum. The ion, X +, will travel through the mass spectrometer just like any other positive ion - and will produce a line on the stick diagram. These uncharged particles will simply get lost in the machine - eventually, they get removed by the vacuum pump. Only charged particles will be accelerated, deflected and detected by the mass spectrometer.
#Mass spec mass finder free#
The uncharged free radical will not produce a line on the mass spectrum. The simplest case is that a molecular ion breaks into two parts - one of which is another positive ion, and the other is an uncharged free radical. The molecular ions are energetically unstable, and some of them will break up into smaller pieces. That's one half of what was originally a pair of electrons - the other half is the electron which was removed in the ionization process. The dot in this second version represents the fact that somewhere in the ion there will be a single unpaired electron. This ion is called the molecular ion - or sometimes the parent ion and is often given the symbol M + or. These electrons have a high enough energy to knock an electron off an organic molecule to form a positive ion. When the vaporized organic sample passes into the ionization chamber of a mass spectrometer, it is bombarded by a stream of electrons.


 0 kommentar(er)
0 kommentar(er)
